HYDROGEN CHLORIDE
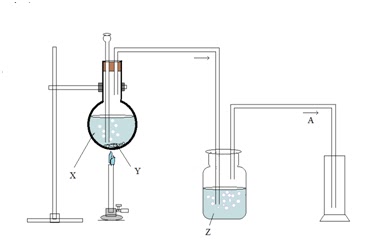
The diagram demonstrates the laboratory preparation
of HCl gas.
a) i)
Name the solid reactant Y taken in the round bottom flask.
ii) Name the liquid reactant added to Y using
thistle funnel.
iii) State the method used to collect HCl gas prepared in the above process. And give
reason for the same.
iv)
Give the identification test for HCl
gas.
b) Give
reason:
i) The temperature should be maintained below 2000C
in the preparation of HCl in the above process.
ii) CaO (quick lime) is not used as a drying agent
in the above process.
c) Write
balanced equations :
i)
Laboratory preparation of HCl gas.
ii)
Reversible thermal decomposition of HCl
gas.
iii)
Reaction between HCl gas and Iron.
No comments:
Post a Comment