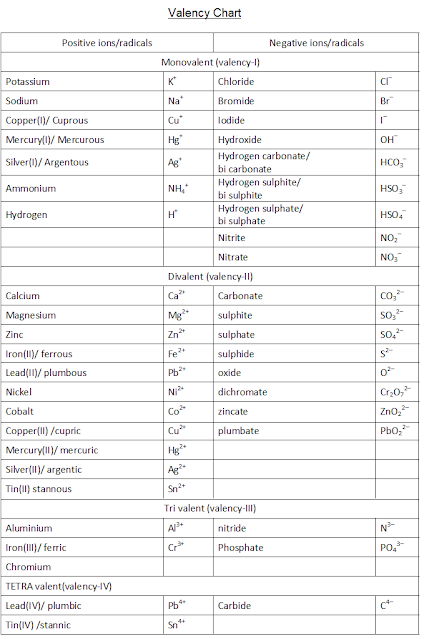
Valency chart - for writing chemical formulas of chemical compounds - criss-cross method
This valency (oxidation states) chart helps in writing the molecular formula of chemical compounds
|
Positive ions/radicals |
Negative ions/radicals |
||
|
Monovalent (valency-I) |
|||
|
Potassium |
K+ |
Chloride |
Cl– |
|
Sodium |
Na+ |
Bromide |
Br– |
|
Copper(I)/
Cuprous |
Cu+ |
Iodide |
I– |
|
Mercury(I)/
Mercurous |
Hg+ |
Hydroxide |
OH– |
|
Silver(I)/
Argentous |
Ag+ |
Hydrogen
carbonate/ bi carbonate |
HCO3– |
|
Ammonium |
NH4+ |
Hydrogen
sulphite/ bi sulphite |
HSO3– |
|
Hydrogen |
H+ |
Hydrogen
sulphate/ bi sulphate |
HSO4– |
|
|
|
Nitrite |
NO2– |
|
|
|
Nitrate |
NO3– |
|
Divalent (valency-II) |
|||
|
Calcium |
Ca2+ |
Carbonate |
CO32– |
|
Magnesium |
Mg2+ |
sulphite |
SO32– |
|
Zinc |
Zn2+ |
sulphate |
SO42– |
|
Iron(II)/
ferrous |
Fe2+ |
sulphide |
S2– |
|
Lead(II)/
plumbous |
Pb2+ |
oxide |
O2– |
|
Nickel |
Ni2+ |
dichromate |
Cr2O72– |
|
Cobalt |
Co2+ |
zincate |
ZnO22– |
|
Copper(II) /cupric |
Cu2+ |
plumbite |
PbO22– |
|
Mercury(II)/
mercuric |
Hg2+ |
|
|
|
Silver(II)/
argentic |
Ag2+ |
|
|
|
Tin(II) stannous |
Sn2+ |
|
|
|
Tri valent (valency-III) |
|||
|
Aluminium |
Al3+ |
nitride |
N3– |
|
Iron(III)/
ferric |
Fe3+ |
Phosphate |
PO43– |
|
Chromium |
Cr3+ |
|
|
|
TETRA
valent(valency-IV) |
|||
|
Lead(IV)/
plumbic |
Pb4+ |
Carbide |
C4– |
|
Tin(IV) /stannic |
Sn4+ |
|
|
 |
| valency chart for writing chemical formulas |


1 comment:
Great chart
Post a Comment