ELECTROLYSIS - CLASS 10 ICSE - QUICK REVISION TEST
Subscribe to ICSE CHEMISTRY for Chapter-wise Notes Practice Papers Work sheets pdf files
1. A solid substance is composed of only ions. It acts as a ___________ (strong/ weak/ non) eelctrolyte.
2. The flow of current in an electrolyte ____________ (increases/ decreases) with the increase in the concentration of free ions in it.
3. An electrolyte which conducts electricity in large amounts in its aqueous solution undergoes _______ (complete/partial/no) dissociation.
4. An example of a polar covalent compound which acts as an electrovalent compound in its aqueous solutions and is a strong electrolyte.
5. Pure water does not conduct electricity hence this acid is added to water before its electrolysis.
6. The separation of ions of an electrovalent compound when it is dissolved in water is called ___________ (dissociation/ ionization).
7. Oxygen is released at _________ (anode/cathode) in the electrolysis of acidified water.
8. Metal is deposited at ________ (anode/cathode) during an electrolysis of a salt solution.
9. ____________ electrode accepts electrons from _______ hence it is called oxidation electrode.
10. ________ migrate towards anode and ______ migrate towards cathode in electrolysis process.
11. The ease of reduction of a metal ion ____________ as we move down the electrochemical series.
12. This ion is called spectator ion in the electrolysis process.
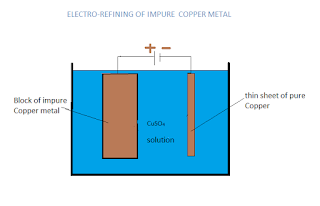
13. The following diagram shows the electrorefining of impure copper metal.
i) State one appropriate observation in this.
ii) Why a pure thin sheet of Copper is taken as cathode.
iii) Where are the impurities collected in this process.
No comments:
Post a Comment