ICSE CLASS 10 CHEMISTRY
ICSE EXAMINATION
Class-X Marks:80
Subject: Chemistry Time:2Hrs.
Answers to this paper must be written on the paper
provided separately.
You will not be allowed to write during the first 15
minutes.
This time is to be spent on reading the Question
Paper.
The time given at the head of this paper is the time
allowed for writing the answers.
Section I is compulsory. Attempt any four questions
from Section II. The intended marks for questions or parts of questions are
given in brackets ( ).
Section I (40
Marks)
Answer all the
questions
Question 1
a) Select the
correct answers from A, B, C or D for the following statements: 5M
i)
An
element ‘X’ belongs to Group 2 of period 3. Then ‘X’ has
A) 3 valence
electrons and 2 shells
B) 2 valence
electrons and 3 shells
C) 2 valence
electrons and 2 shells
D) 1 valence
electron and 2 shells
ii)
Which
of the following compounds involves formation of coordinate bonding?
A)
CCl4 B)
H2 C)
HCl D) NH4Cl
iii)
Which
of the followings not an alloy of Copper?
A)
Brass B) Bronze C) Solder D) Duralumin
iv)
The
catalyst used in the preparation of Ammonia in Haber’s process:
A) Iron B)
Platinum C) Silver D) Nickel
v)
Which
of the following properties doesnot describe the properties of alkenes?
A) They are
unsaturated hydrocarbons
B) They
decolourise Bromine water
C) They can
undergo addition reactions as well as substitution reactions.
D) They undergo
combustion reaction with Oxygen forming Carbon dioxide and Water.
b)
Fill in the blanks:- 5M
i)
Metals
tend to __________ (lose/gain) electrons.
ii)
_______________
(Electrorefining / electroplating) makes an article attractive.
iii)
Cold
dilute Nitric acid reacts with Copper metal to form ___________ (Nitrogen
dioxide/Nitrous oxide/Nitric oxide).
iv)
Sodium
ethanoate reacts with Soda lime to release ____________ (Methane/Ethane) gas.
v)
Cations
formed by _________ electrons are reduced at ___________. (gaining, losing,
cathode, anode)
c)
Name the following. 5M
i)
A
metal present in period 3 with 1 valence electron.
ii)
The
element with highest electronegativity.
iii)
A
covalent compound which behaves like an electrovalent compound in its aqueous
solution.
iv)
An
acid which is present in vinegar.
v)
The
explosive liquid formed when ammonia reacts with excess Chlorine gas.
d) Distinguish between the following pair of compounds using a common reagent. 5M
i)
Ethene
and Ethyne
ii)
Dil.
Hydrochloric acid and dil. Nitric acid.
iii)
Sodium
chloride and Zinc chloride.
iv)
Calcium
carbonate and Calcium nitrate
v)
Magnesium
sulphate and Magnesium chloride.
e) Give reason why: 5M
i) The oxidizing power of elements
increases across a period from left to right.
ii) Dilute H2SO4
is preferred dil. HNO3 to dilute water before its electrolysis.
iii) In the electrolysis of Alumina in
Hall Heroult’s process the electrolyte is covered with powdered coke.
iv) Ammonium nitrate is not used in the
preparation of Ammonia gas.
v) Alkenes are more reactive than
alkanes.
f) State your observations : 5M
i) At anode when aqueous solution of
Copper sulphate is electrolysed using copper electrodes.
ii) Copper carbonate is heated in a hard
test tube.
iii) A few drops of dil. HCl is added to
Silver nitrate solution followed by the addition of Ammonium hydroxide
solution.
iv) Ammonium
hydroxide is added to Lead nitrate solution in small amounts then in
excess.
v) Sodium
chloride crystals are heated with conc. Sulphuric acid.
g)
Select the suitable word from the list given in brackets for each of the
following statements. 5M
(Sodium
chloride, Magnesium chloride, K+, Lead chloride, PbO, Cu2+,
FeO)
i)
A
deliquescent substance.
ii)
A
chloride insoluble in cold water but soluble in hot water.
iii)
An
amphoteric oxide.
iv)
The
ion which could be discharged most readily at cathode.
v)
A
soluble salt.
h) (NH4)2Cr2O7
N2 + 4H2O + Cr2O3 5M
(at.
wt. H-1, N-14, O-16, Cr-52)
A) If 63 g of
Ammonium dichromate is heated:
i) Calculate the
volume of Nitrogen gas produced.
ii) The number
of moles of Ammonium dichromate taken.
iii) The mass of
Cr2O3 formed.
B) i) Calculate the amount of (NH4)2Cr2O7
to be taken to produce 44.8lit. of Nitrogen gas.
ii) Find the number of moles of
Nitrogen gas produced.
Section
II (40 Marks) Answer
any four of the following questions
Question 2 5+5
a) A, B, C and D are the elements with valence
electrons 6, 3, 4 and 1 respectively. Answer the following:
i)
Nature of the bonding between C and A.
ii)
Formula of the compound between B and A.
iii)
Nature of the bonding between D and A. and draw the electron dot structure of
that compound.
iv) If
A is a non metal containing diatomic molecule then write balanced equation for
the reaction between A and B.
v) If they all belong to same period which of them
is most electro negative?
b) Salts
A,B,C,D and E undergo the following reactions i-v respectively. Then find the
anions present each salt.
i)
When AgNO3 is added to the solution of A, a white precipitate is
formed which is insoluble in dil. HNO3.
ii) Addition of dil. HCl to B produces a gas which
turns Lead acetate paper black.
iii) When a freshly prepared solution of FeSO4
is added to a solution of C and conc. H2SO4 is gently added , a brown ring is formed.
iv) When dil. Sulphuric acid is added to D a gas is
produced which turns lime water milky and turns acidified K2Cr2O7
to clear green.
v) Dil. Hydrochloric acid is added to a solution of
‘E’. A gas is release which turns lime water milky but has no effect on
acidified K2Cr2O7.
Question 3 5+3+2
a)
IA
|
IIA
|
IIIA
|
IVA
|
VA
|
VIA
|
VIIA
|
0
|
||||||||||||||||||
1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
13
|
14
|
15
|
16
|
17
|
18
|
|||||||||||
1
|
A
|
||||||||||||||||||||||||
2
|
E
|
Q
|
D
|
X
|
Z
|
||||||||||||||||||||
3
|
C
|
H
|
T
|
Y
|
J
|
||||||||||||||||||||
4
|
M
|
||||||||||||||||||||||||
i) State the number of shells present in ‘C’.
ii) Formula of the compound between ‘Q’
and ‘X’.
iii) What type of bonding is possible
between A and Z.
iv) Which one has the highest
electronegativity among the elements belonging to Period 3. (from the given
table).
v) Identify the metalloids in the table.
b) Give one suitable term for the
following statements.
i) The energy required to remove one
valence electron from a gaseous atom.
ii) The tendency of an element to
attract bond pair of electrons to itself.
iii) Physical and chemical properties of
elements are periodic functions of their atomic number.
c)
i) Name the process in which Sulphuric
acid is manufactured in large amounts.
ii) Give one chemical test for dil. Sulphuric
acid.
Question 4
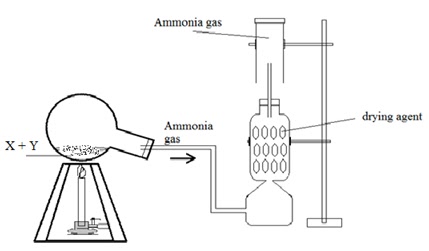
a) The
following diagram shows the laboratory preparation of Ammonia gas. 5+3+2
i) Identify X and Y.
ii) State the nature of the gas prepared.
iii) Give a balanced equation for the above preparation.
iv) Name the drying agent.
v) State how will you find out that the jar is full of Ammonia
gas?
b)
i) In
laboratory preparation of Hydrochloric acid , HCl gas is dissolved in water
using funnel arrangement. Give any two reasons for the same
ii) Write
balanced equation for the laboratory preparation of HCl gas.
c) Write
any two reactions which show the reducing property of Ammonia gas.
Question
5 2+4+2+2
a)
i) What volume of Oxygen is required to burn completely 90dm3 of
Butane under similar conditions.
2C4H10
+ 13O2 à 8CO2 + 10H2O
ii)
The mass of 5.6dm3 of a gas at STP is 12g. Calculate the relative
molecular mass of the gas.
b)
i) An organic compound has vapour density 94. It contains C=12.67%. H=2.13% and
Br=85.11%.
Find the molecular formula of the given organic compound. (C-12,H-1,Br-80)
ii)
Hydrogen gas contains X number of molecules under certain pressure and
temperature. Find the number of molecules that the same volume of Oxygen
contains under same conditions.
c)
Write balanced equations for the following reactions:
i)
ZnO reacts with Sodium hydroxide
ii)
Ferric chloride from Iron
d)
Name the gases released in the following cases:
i)
dil. HCl is added to a metal carbonate
ii)
An alkali is heated with an Ammonium salt.
Question
6 3+3+2+2
a) i)
A solution of Silver nitrate is a good electrolyte but not used as electrolyte
for electroplating with Silver. Why?
ii) Write balanced electrode equation at
cathode during electrolysis of Copper sulphate using inert electrodes.
iii) Name the products formed during the electrolysis
of acidified water. (mention the electrode and write answer)
b) i) State any two conditions during electro plating.
ii) State one
difference between a strong electrolyte and a weak electrolyte.
c) Answer
the following questions pertaining electrolysis of Alumina.
i) Name the process.
ii) What is the significance of the Aluminium compound other
than Alumina in this process?
d) Name
the following:
i) The chemical added to the ore to get rid of impurities.
ii) An allotrope a non
metal which is a good conductor of electricity.
Question
7 2+3+2+3
a) Write
the branched structures of the following compounds:
i) 1,2-di chloro ethane ii) Di methyl ether
b) i)
Give reason why Ethene under goes addition
reactions but Ethane does not.
ii) State your observation when is Ethene passed
through Bromine water.
iii) Name the process of removal of Carbon dioxide
from a carboxylic acid.
c) Write
balanced equations for the following reactions:
i) Dehydration of Ethyl alcohol at 1700C
using a dehydrating agent.
ii) Esterification of Acetic acid.
d) Copy and complete the following table:
Salt Solution
|
On addition of
NH4OH in small quantity
|
On addition of
NH4OH in excess
|
Cation present
in the salt solution
|
A
|
Fe2+
|
||
B
|
Gelatinous
white ppt.
|
||
C
|
Inky blue
solution
|
No comments:
Post a Comment