CLASS 10 Chemical Bonding ICSE notes
Covalent
bond:
The bond formed by the mutual sharing of electron
pairs between the given pairs of atoms (of same or different kind) of non
metals.
Covalent compound (molecule) : The compounds
(molecules) formed as a result of mutual sharing of electrons between the atoms
are called covalent compounds.
Covalency: The number of electron pairs that an atom
shares with other atom/s to get stable electronic configuration.
Formation covalent molecules - Electron dot representation
·
The element which has 7 valence
electrons (i.e. short of one electron for octet configuration) contributes one
electron and shares one pair of electron with other atom.
Cl - 2,8,7 Cl
- 2,8,7 Chlorine molecule single covalent bond
·
The element having 6 valence electrons
(short of 2 electrons) contributes 2 electrons hence shares two pairs of
electrons.
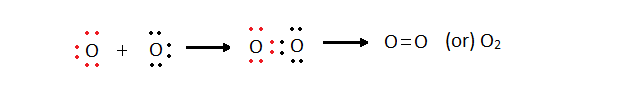
Oxygen Oxygen Oxygen molecule Double bond
2,6 2,6
·
Similarly the element with 5 valence
electrons contributes 3 electrons and shares three pairs of electrons.
Nitrogen Nitrogen Nitrogen molecule
2,5 2,5 Triple
bond
·
Formation of Hydrogen molecule
·
Formation of Methane molecule (CH4)
·
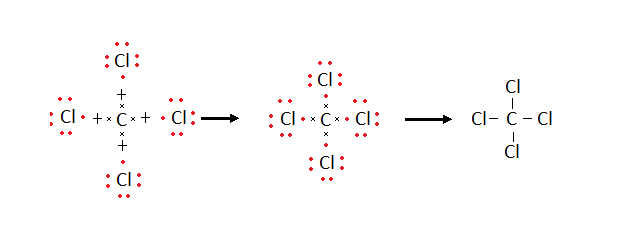
Formation of Carbon tetra chloride molecule (CCl4)
Formation of Hydrogen chloride molecules (HCl)
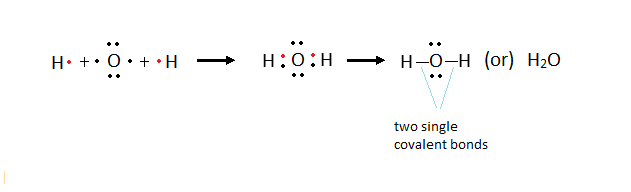
· Formation of Water molecule:
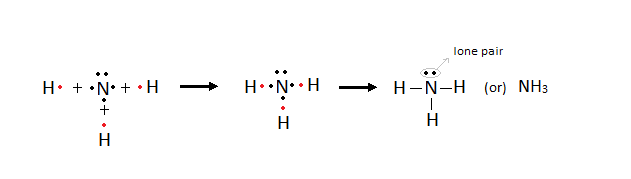
Ø Lone
pair: The pair of electrons which is not involved in any
bond formation.
for complete chapter pdf file click here class 10 Chemical Bonding pdf
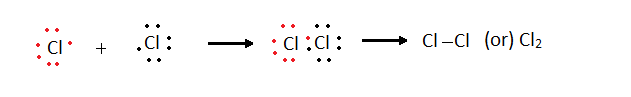



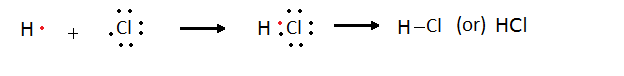
No comments:
Post a Comment