ICSE CHEMISTRY CLASS 10 METALLURGY - QUICK REVISION TEST
ONLINE CLASS ON ZOOM
Duration 5days
Quick Revision and worksheets
Fee 2000/-
Contact 8897306498
Contact 8897306498
1. Name the metal whose magnetic ore is separated from non-magnetic impurities using magnetic separation method.
2. What is meant by 'Mineral' ? State the names of two minerals and their formula.
3. What are the impurities associated with an ore called?
4. Name the process in which a metal sulphide ore is converted to Metal oxide by heating it in presence of air.
5. Name an ore which is concentrated by Froth Flotation method.
6. Name a mineral of metal which can be converted to the respective metal oxide by Calcination process.
7. Give two examples of reducing agents used in Metallurgy to convert a metal oxide to metal.
8. Name any two ores of Aluminium.
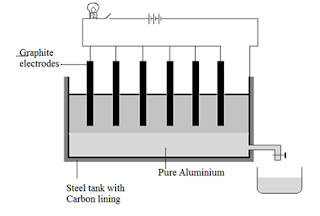
9. Answer the following questions pertaining to the diagram given below:
i) Name the process illustrated by the given diagram.
ii) State the composition of the electrolyte used in this process.
iii) Name the material used as cathode here.
iv) Write balanced Cathode reaction.
v) Give reason why the Coke powder is sprinkled on the electrolyte.
vi) What is the purpose of adding Cryolite to the electrolyte.
10. Write the balanced equations involved in Baeyer's process.
Solution - Metallurgy Quick Revision Test:
1. Iron
2. A naturally occurring compound which is associated earthly impurities.
example : Hematite Fe2O3, Magnetite - Fe3O4
3. Matrix or Gaunge
4. Roasting
5. Zinc Blende
6. Calamine - ZnCO3
7. Coke, Carbon monoxide, Hydrogen gas etc.
8. Bauxite, Cryolite
9. i) Hall-Heroult's Process
ii) Bauxite, Cryolite, Flourspar in the ratio 1:3:1
iii) Carbon
iv) 2Al3+ + 6e –
→ 2Al
v) To prevent heat loss by radiation and reduces the burning of Graphite electrodes in presence of atmospheric oxygen
vi) Cryolite reduces the fusion point of Alumina
10.
Al2O3.2H2O +
2NaOH →
2NaAlO2 + 3H2O Temp: 150-200 degree Celsius
NaAlO2
+ 2H2O → NaOH + Al(OH)3 Temp: 50-60 degree Celsius
2Al(OH)3 → Al2O3 + 3H2O Temp: 1100 degree Celsius
No comments:
Post a Comment