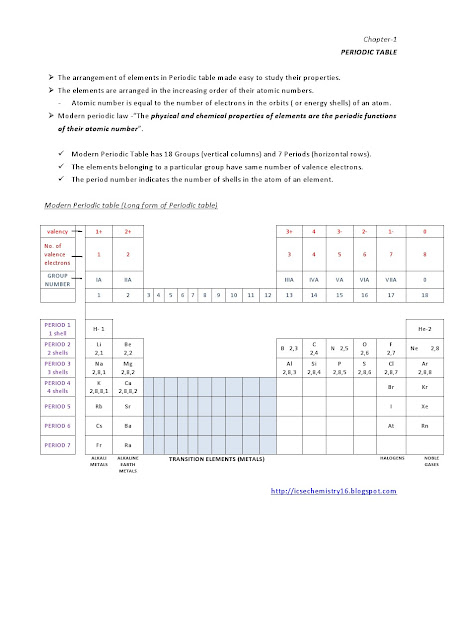
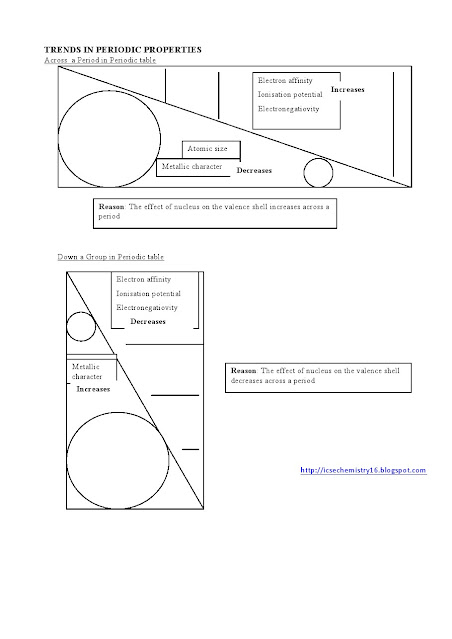
Class 10 ICSE CHEMISTRY questions on ACIDS AND BASES - 1
Name the following.
1. The
positively charged ions produced when an acid is dissolved in water.
2. The
ions responsible for the acidic nature of an acid.
3. The
gas which is released when a dil. acid reacts with a metal carbonate.
4. The
gas liberated when a dilute acid reacts with a metal sulphide.
5. The
gas liberated when a dilute acid reacts with a metal sulphite.
6. A
water soluble compound which gives hydroxyl ions as the only negatively charged
ions.
7. The
acid present in vinegar.
8. The
acidic oxide which dissolves in water to produce Sulphuric acid.
9. The
number of H+ ions that can be produced per one molecule of an acid
in its aqueous solution.
10. The
number of replaceable hydroxyl ions per one molecule of the base.
11. The
acidity of Aluminium hydroxide.
12. The
acidity of Acetic acid.
13. The
volatile acid produced when a non volatile acid is heated with NaCl.
14. The
non volatile acid which is prepared by the oxidation of a non metal using a
volatile acid.
15. The
reddish brown precipitate formed when Sodium hydroxide is added to Ferric
sulphate.
16. The
acid used in eye wash.
17. The
acid used as food preservative.
18. The
acid used to remove ink stains.
19. The
acid used in pickling metals.
20. The
base used as antacid.
21. The
base used to remove grease stains.
22. The
gas released when Ammonium salts are heated with an alkali.
23. A
mono basic hydracids.
24. The
basic oxide dissolves in water to produce Sodium hydroxide.
25. A
compound which when dissolved in water produces hydronium ions as the only
positively charged ions.
26. An
acid can give only a normal salt with a base then its basicity is ____________.
27. An
acid responsible for acid rain.
28. The
base produced when Lead nitrate is heated.
29. The
acids which when dissolved in water dissociate completely and produce a high
concentration of Hydronium ions.